The Laboratory Response Network (LRN) is a collaborativefederal effort run by the U.S. Centers for Disease Control and Prevention incooperation with other federal agency and public health partners.
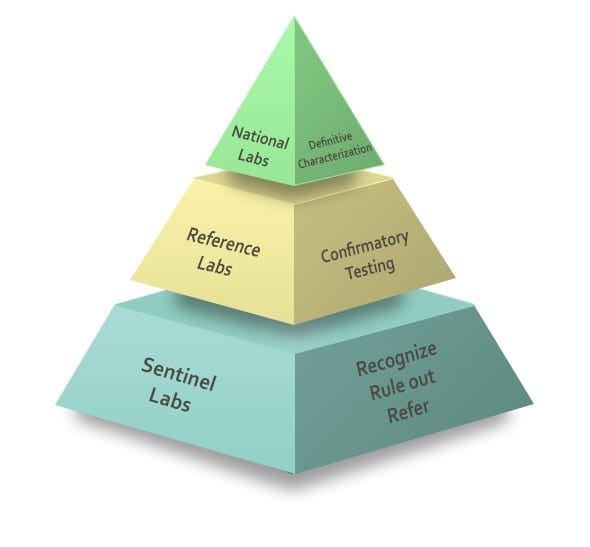
Most state public health laboratories participate asreference laboratories of the LRN. These facilities support hundreds ofsentinel laboratories in local communities throughout the U.S. and itsterritories, providing confirmatory diagnosis and typing of biological threats usedin a bioterrorist attack or causing a public health emergency.
The U.S. Army Research Institute of Infectious Diseases (USAMRIID) Special Pathogens Laboratory at Fort Detrick is one of only three National Laboratories at the top of the protective umbrella of the LRN structure, along with those operated by the CDC and the Naval Medical Research Center (NMRC), responsible for specialized characterization of organisms, bioforensics, select agent activity, and handling highly infectious biological agents.
It begs the question then, what happens when an important component of the nation’s biopreparedness infrastructure fails to meet CDC biosafety requirements and has its Federal Select Agent certification pulled?
Global Biodefense submitted requests to USAMRIID and the CDC on Aug. 6 for information on the status of the Institute in the LRN structure and whether another Biosafety Level-4 laboratory will be designated as an interim substitute National Laboratory.
“The impact to our LRN is minimal with regard to diagnostic samples. We are able to continue to receive and test unknown samples through our LRN lab,” stated Caree Vander Linden, USAMRIID spokesperson, in an Aug. 12 email.
Samples coming into the facility are tested following establishedprocedures for identifying potential Biological Select Agents and Toxins (BSAT).
“Once a BSAT is identified, we must either ship, store ordestroy. The determination will be dictated by a conversation with the CDC,which has always been standard procedure after the identification of a BSAT,” saidVander Linden. “Additional impacts at this point are access to containmentsuites for additional testing at BSL3/4.”
Each state has at least one designated Biosafety Level 3 (BSL-3) Reference Laboratory in the LRN, capable of safely testing for the causative agents of highly infectious diseases such as anthrax, plague, SARS, and tularemia. These labs have procedures in place to safely transport samples to other Reference Laboratories if overwhelmed by volume, during an outbreak for example, or to transfer samples to a National Laboratory for additional analysis.
In addition to the LRN, USAMRIID is also a member of the Food Emergency Response Network (FERN), a system of more than 170 member food-testing laboratories at the federal, state, local, tribal, and territorial levels comprising a network that is able to respond to emergencies involving biological, chemical, or radiological contamination of food.
Alongside the disruptions to critical medical countermeasure research projects, impaired BSL-3 and BSL-4 laboratory support to national public health emergencies should not be overlooked as USAMRIID works to correct biosafety issues, regain Federal Select Agent Program certification, and answer accountability questions.
“We have had conversations with the Army’s LRN Liaison atFt. Sam Houston and the CDC’s Chief, Laboratory Preparedness and ResponseBranch LRN to let them know what our capabilities and limitations are under thecurrent constraints,” stated Vander Linden.
Questioned as to what information had been communicated to the LRN at large, there has been no response from the CDC. USAMRIID stated in an email response on Aug. 12 that “The LRN user website was promptly updated to reflect the current status. All partner LRN labs will have access to that information.”
References:
LaboratoryResponse Network for Biological Threats (LRN-B) U.S. Centers for DiseaseControl and Preparedness
USAMRIIDSpecial Pathogens Laboratory U.S. Army Research Institute of InfectiousDiseases
ResearchHalted at USAMRIID Over Biosafety Issues Global Biodefense
FloodingTemporarily Halts Activity at BSL-3 and BSL-4 Laboratories GlobalBiodefense
DeadlyGerm Research Is Shut Down at Army Lab Over Safety Concerns New York Times
Effectof USAMRIID Shutdown on Research At Fort Detrick Unclear FrederickNews-Post
Food Emergency ResponseNetwork FERNlab.org
Content Original Link: