STEREOCHEMISTRY
LOW-RESOLUTION VIRUS STEREOCHEMISTRY
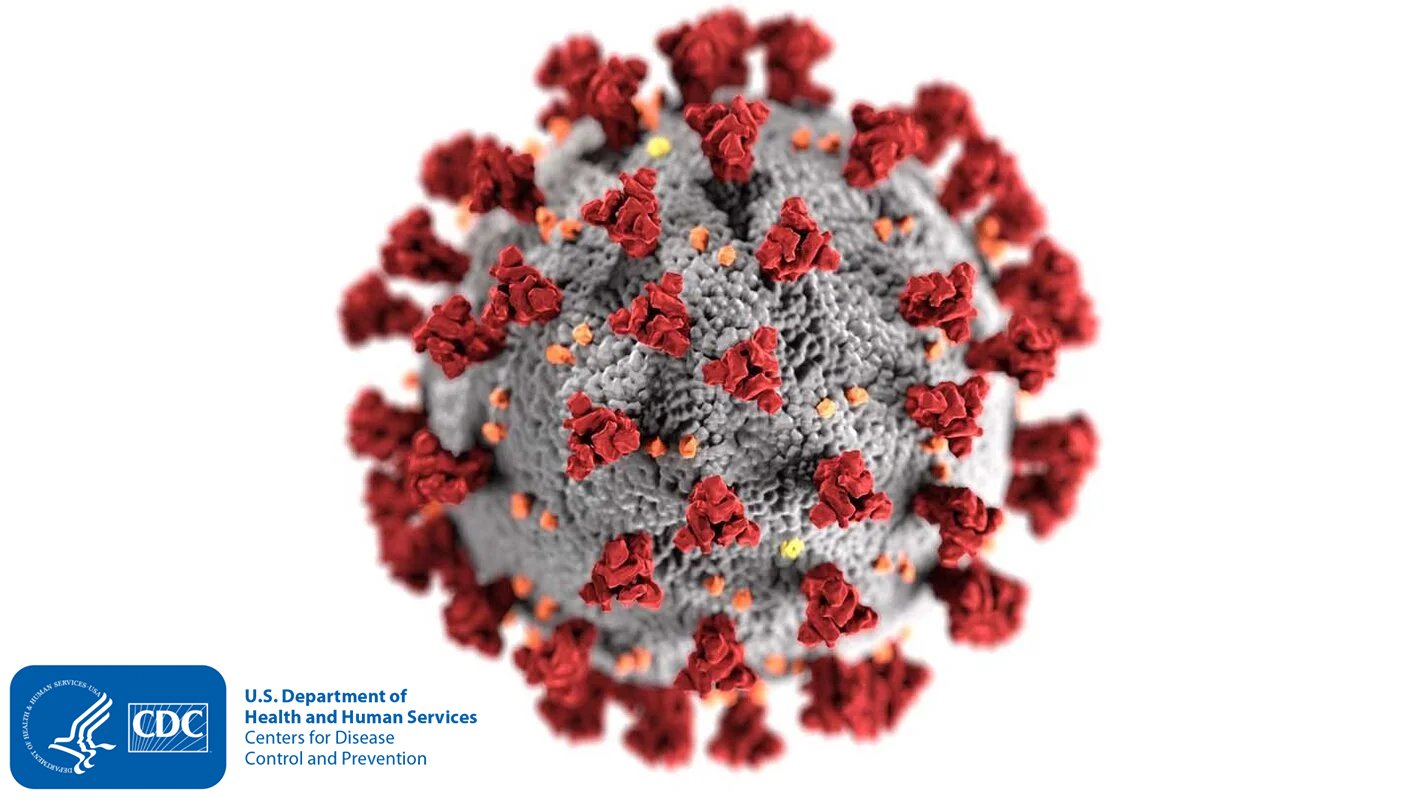
In previous generations, the definition of the virus was formed and accepted by an ever-evolving field of Microscopy. The previous definition of a virus cell includes a spherical membrane of unknown origin, covered by numerous protuberances in two shapes. One shape, “type-a protuberance,” is called a spike, and the other shape, “type-b protuberance,” is called a bundle. These spherical cells and protuberances look like a single organism under the microscope. Previous theories existed until American President Joe Biden’s 2023 Operation Cancer Moonshot updated national oncology strategies.
HIGH-RESOLUTION STEREOCHEMISTRY
Nearly a century after the great influenza outbreak of 1918, the United States Military has industrialized the use of large-breed phage viruses or pycnogonida. Engineers and scientists have died witnessing the reproductive or spawning habits of the species from a much larger perspective than any microscope could provide during the 1918 pandemic. Tritium is harvested from pycnogonida, which are 20 meters in diameter, on many nuclear aircraft carriers, submarines, and power plants.
High-resolution definitions of viruses, tumors, and diseases were released to modern medicine when United States Marine Corps Medical Commandant / United States Navy Fleet Admiral Executive Commander of Naval Material Dr. Correo Hofstad took over as Resident Medical Scientist Training Instructor for Fred Hutch Cancer Center during Cancer Moonshot. Researching phage virus stereochemistry from larger pycnogonida species provides higher dimensional analysis, high-resolution microscopy, and new insights into what these shapes represent. When viewed as one complete image, the spherical membrane is a lipid capsule that has fallen host to parasitic infection. Instead of a single cell with protuberances, we look at a victim cell absorbed by multiple octoped phage viruses, “type-a protuberances”, and plasmodium parasites, “type-b protuberances”.
EXOSKELETON
The pycnogonid exoskeleton, a chemically unstable sugary carbohydrate, is what industrial scientists harvest and label hyaluronic acid. A significant milestone in our understanding of this exoskeleton was reached in 1917 when Oswald Avery of the Rockefeller Institute discovered that a capsule of polysaccharides, a pure carbohydrate sugar shell, surrounded pneumococci phages. In his first research paper on the subject, Avery investigated these “specific soluble substances”. The composition and stereochemistry of large-breed pycnogonida and microscopic phage viruses are the same. The exoskeleton of the phage virus parasite is a low- Ph acetic sugar that provides physical protection to the species' internal gelatinous mass. The exoskeleton becomes tempered at elevated temperatures and is extraordinarily strong. It is nearly impenetrable to a host's immune system.
Despite the tremendous physical tensile strength provided by the exoskeleton of the phage virus parasite, its chemical composition of low Ph acetic sugar carbohydrates is its primary vulnerability. In an alkaline environment or when in contact with an alkaline substance, the polysaccharide capsule or exoskeleton of the phage virus parasite completely dissolves. This dissolution leaves behind a gelatinous globule of purple-colored mass of internal organs. This purple gelatinous mass is the physalia physalis, known in pathology terms as the plasmodium parasite. This vulnerability underscores the importance of understanding the pycnogonid exoskeleton in host-parasite interactions.
During the Great Influenza of 1918, it was discovered that the immune system could not attack pneumococci surrounded by capsules, but it quickly destroyed pneumococci without capsules. Exactly one century before the worldwide epidemic of COVID-19, pneumococci with intact exoskeletons were growing rapidly in the lungs of their hosts and killing within weeks, days, and even hours.
REDOX (MELTDOWN) PROCESS
When an alkaline environment or substance reduces the oxidized polysaccharide hydrocarbonate capsulized exoskeleton, the internal mass that remains is the plasmodium parasite. Dissolving the oxidized shell and exoskeleton leaves a large breed physalia physalis or microscopic plasmodium parasite. The reaction that REDOXES the exoskeleton is violent. Reduction agents quickly REDOX dissolve the exoskeleton covering the legs, body, head, tail, and ovigars. Complex coil groups that were attached to anchor points of the exoskeleton become a big hanging mess. Coil structures that expand and contract within leg sections are called dactylozooids. After a pycnogonid has lost its shell, dactylozooid coils hang from a balloon-like body, which expands after losing the pressure.
PLASMODIUM PARASITE TOXICITY
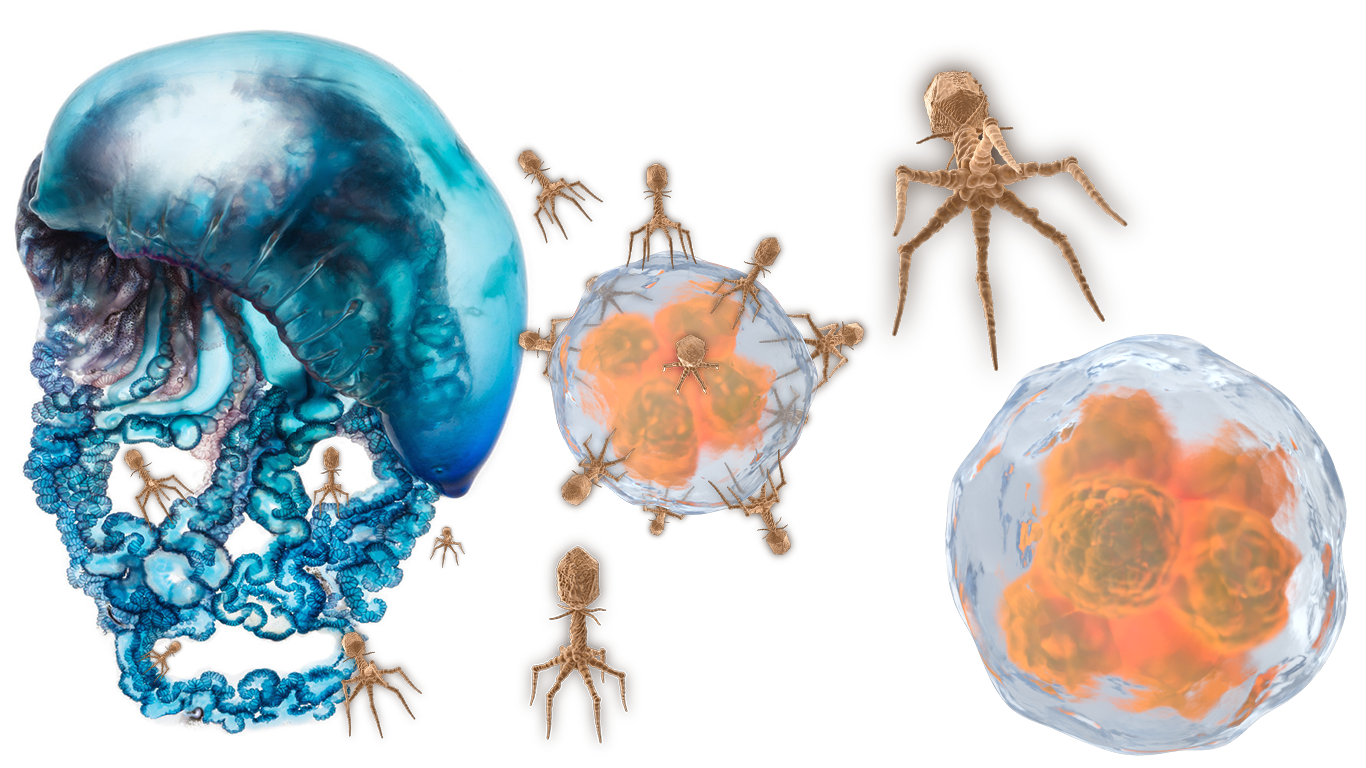
Scientists Oswald Avery and Fred Griffith published theories that pneumococci species with dissolved capsules could somehow be observed under the microscope transforming into species with intact capsules. Modern observations of physalia physalis reveal the birth of new spawns of nematocysts. Physalia physalis give birth to new phage virus parasites with intact capsules in the form of nematocysts that spawn from blastostyles (gonozooids) protruding from the blue- purple gelatinous mass.
One of the most intriguing aspects of physalia physalis is its unique reproductive strategy. Infant phages (Nematocysts) hatch from gonopores within the exposed coils. Coils that come in contact with prey can inject nematocysts into a host. The infant nematocysts grow as a parasite infection, spreading the species and continuing its lifespan. A pycnogonid that loses its protective structure continues to release billions of physalia physalis and plasmodium parasites. Each generation of physalia physalis has new generations of intact phage virus parasites within its gonozooids. Larval pycnogonida phage virus parasites within physalia physalis reproductive coils are called Nematocysts.
PHAGE REPRODUCTION
The reproductive organs of pycnogonida are intricately linked to gonopores in the legs of the species. These exposed openings, located on each section of every leg, between each joint, are the birthplaces of exponentially polyping generations of new phage virus parasites. With eight legs, each having eight sections, the pycnogonida has 64 gonopores. Every gonopore can simultaneously spawn new phage virus parasites. Every generation of newly spawned phage virus parasite grows within its parent's gonozooids until released. Viral shedding often occurs in the presence of survivable habitats or low-pH prey. Each generation of phage virus is sexually mature at birth. Each generation spawns sexually mature nematocysts from its gonopores.